Petroleum
First thing to remember about petroleum toxicity is that crude oil and most refined petroleum products are not "chemicals" or "pure substances" in the the thermodynamic sense. Rather they are mixtures of chemicals having varying molecular size and structure.
 |
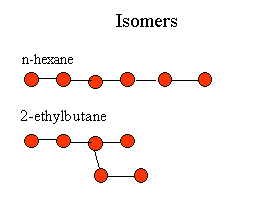
The number of possible isomers increases with number of carbons. The toxicity varies enormous between isomers. n-hexane is quite toxic, I doubt 2-ethylbutane is toxic except at very high doses. |
Most mixtures vary with time and place. That is, the crude oil that comes from an oil well will have a different chemical composition as the well produces over the years. This composition will likely be different than the composition from even nearby wells. (Often oilfields will have certain characteristics, "fingerprints," different than other fields, but these are based on certain chemicals, but all these too vary through the years.) Refined products can best be thought of as a set of specifications rather than a chemical. The API or the various users set specifications for specific gravity, viscosity, octane ratings, and so on. So does the EPA. Benzene content of gasoline, for example, might vary from 2.5% to 15%. The EPA wants the benzene content of gasoline reduced to below 1%.(I remember reading of a gasoline that was 30% benzene, but I couldn't find the reference. The German word for gasoline is "benzin.")
Crude oil when it comes out of the ground is a mixture of hydrocarbons and other organic chemicals, water, sand, and solids. The hydrocarbons often include volatile hydrocarbons and these are sometime called gas or natural gas. In so far as practical, the producers of crude oil remove the water, sand, and gas near the wellhead because it is costly to transport it and they are allowed to reinject these into nearby wells.
Crude oil usually contains some sulfur containing compounds such as hydrogen sulfide, H2S is a gas often associated with crude oil. It is toxic enough, but we have discussed that earlier. Crude will sometimes have some oxygen containing compounds such as aldehydes.
It is sometimes useful in environmental work to categorize the hydrocarbons by the number of carbons in the typical molecules By that method C1 (methane) through C3 (propane) are gases. C4 and C5 are gas liquids. C1 through C5 are volatile. C6 through C10 are gasoline range organics (GRO, aka "semi-volatiles"), C10 through C25 as diesel range organics (DRO), and C25 through C36 as residual range organic (RRO). You will often hear these terms because they are handy for many purposes, but not toxicology.
For toxicity, it is handy to divide petroleum into: light aromatics (BTEX, as we discussed earlier), light aliphatics, PAHs, heavy aliphatics.
The light aromatics are volatile so they can be absorbed by inhalation, dermal absorption and ingestion. They are toxic, as we discussed earlier. They are "less hydrophobic" than aliphatics with the same number of carbons.
With the exception of n-hexane and a few similar compounds, the light aliphatics are very not toxic, as humans would encounter them. They can be absorbed through the skin and some are irritants. They may be toxic to aquatic life, since they will be absorbed in microorganisms and small animals. These represent lipid centers in the water, and whatever hydrocarbon that is dissolved in water will migrate ("partition") into the animal. While we are there, in large oil spills many small animals will die from gross contamination of their gills. Those effects are obvious and are seldom subjects of toxicology studies.
The PAH's
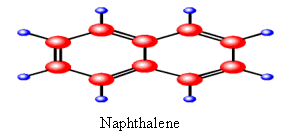 |
The PAHs, naphthalene (2 benzene rings) is a solid but it evaporates. naphthalene is toxic to blood forming organs. |
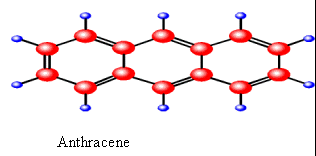 |
Anthracene (three benzene rings) |
 |
and beyond would be solid if they were pure. They are sometimes dissolved in oil. They are heavy tar-like compounds and would not be absorbed if they were room temperature. If heated they can volatilize and be inhaled. In that manner are they carcinogens, at least some of them. Since the chief toxic ingredient of cigarette smoke is a PAH, they have been studied in some detail. The carcinogenicity varies with the ring structure. |
Many PAHs are also irritants. Creosote is a mixture of PAHs dissolved in solvent and applied as a wood preservative. So don't inhale the smoke of burning railroad ties or such. PAHs (sometimes called "creosote" ) are also the chief ingredient inside the chimneys of wood stoves. Again, it is not good to breath.
The heavier aliphatics are generally non toxic. They are chief constituent
of candles. Petroleum jelly (Vaseline) "is a colloidal
system of non-straight-chain solid hydrocarbons and high-boiling liquid hydrocarbons,
in which most of the liquid hydrocarbons are held inside the micelles."
I would not eat Vaseline or candles unless I was very hungry, but you could.
Logic would dictate that we next try to reconcile the toxicity with the common environmental designations, GRO, DRO, and RRO. But there basically is no relation. You would have to analyze the contaminated material for its constituent chemicals, then determine the fate and transport of that chemical to the human. This could be done, but it is impractical, in most cases. If you did do that, you could then make a reasonable determination of the toxicity of the individual chemicals and next make an educated guess about the toxicity of the mixture.