A little organic chemistry
Organic chemistry is the study of carbon compounds. Since life on earth is built of carbon compounds, the field of organic chemistry is very broad. What follows is a brief sketch of some organic chemistry terms that are common in toxicology and hazardous materials. Most chapters in a formal study of organic chemistry start out with explanations of how to name the various compounds. Here just learn some of the major terms, and some examples of more detailed terminology.
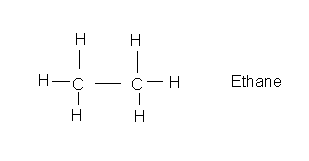
The simplest organic compounds are the hydrocarbons that consist of only carbon and hydrogen.
Here is one that has two carbons:

Here is one with six carbons:
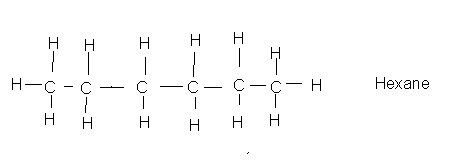
Go here and read the top of this page to see the names of other hydrocarbons in this series (you don't need to download anything) .Parafins
Let's take a moment to review the stick rule and shorthand notation.
We write hexane as:
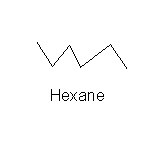
This line representation means that there is a carbon at each apex plus one at each end. And we know the arrangement of the hydrogen atoms from the stick rule.
How about this?

How many carbons does it have . And what is its name
Not all hydrocarbons with eight carbons are octane. The carbons can be linked differently:
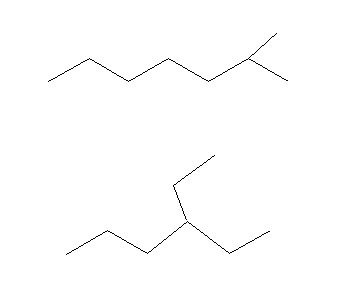
The upper molecule is 1-methylheptane
The lower is 3-ethylhexane
All three arrangements of eight carbons are isomers of each other. Such isomers have the exact same atomic formula and molecular weight, but have slightly different chemical and physical properties. For example their boiling points might be slightly different. The number before the name tells you which carbon on the backbone where the branch takes off.
It is also possible for the end carbons to link to each other, as in:
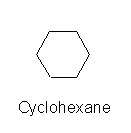
The same rule about the number of hydrogen atoms applies, each carbon has two, but since there are not any end carbons, there are 12 hydrogen atoms attached to cyclohexane. Go back to the web site above and glance at the lower portion of the page. It has lots of examples about how isomers are numbered. Don't bother memorizing from this page, but if you have some familiarity with the naming process, you will be less intimidated by some of the complicated names of environmental contaminants. Here it is again Isomers (Note the page uses a convention to illustrate molecular structure, with wedge shaped lines to indicate if the atoms are in front of or behind the page.)
Hydrocarbons similar to those described above are called alkanes. The trade name for them is aliphatics or parafins.