Hydrocarbons and solvents.
"Do not induce vomiting!!" says the label. Why? Find out below.
Organic solvents are ubiquitous in our industrial society. They are in consumer products like paints, inks, thinners, adhesives, and cosmetics. They are used in industrial process such as metal cleaning and dry cleaning processes. They are the cause of many industrial accidents and poisonings. Most are volatile and their vapors may be inhaled. Most penetrate the skin. Others are ingested with food. I have found it surprising that, although many of these are very volatile compounds, many are persistent in the environment as well.
For convenience we will break these down into aromatic hydrocarbons, aliphatic
hydrocarbons, chlorinated aliphatics, alcohols and glycol and glycol ethers.
Almost forgot chlorinated aromatics, but we got most of them under pesticides,
that's their use rather than solvents, but we'll get PCBs and dioxins in a week
or two.
Aromatic hydrocarbons
The lighter aromatic hydrocarbons are known as BTEX, which stands for benzene and the alky-benzenes, toluene, xylene and ethyl-benzene. Another light aromatic is isopropyl benzene, known as cumene.

We have discussed benzene itself already. All the BTEX compounds are neurotoxic, all cause anesthesia at high doses. Workers at 200 to 300 ppm toluene have impaired reaction times. Very high doses of toluene, such as "glue sniffers" experience, can cause CNS damage. In rats the alkylbenzenes are fatal at about the 4000 to 8000 ppm range. The alkylbenzenes are generally not mutagenic and are not considered carcinogens. The reason for this probably has to do with how they are metabolized. The first step is the oxidation of the alkyl group, not the aromatic ring. All can irritate the URT, eyes, etc. All irritate the skin.
Chlorinated Aliphatics
The chlorinated methanes
| Formula | Proper name, common name | TLV | Organs, notes |
| CH3Cl | Chloromethane, methyl chloride | 50 | CNS, liver, kidney, reproductive |
| CH2Cl2 | Dichloromethane, methylene chloride | 50 | Widely used solvent. CNS, animal carcinogen, produces carbon monoxide thus carboxyhemeoglobin |
| CHCl3 | Trichloromethane, chloroform | 10 | Former anesthetic, CNS, liver, kidney, heart damage, reproductive, animal carcinogen |
| CCl4 | Tetrachloromethane, carbon tetrachloride. | 5 | liver, certain animal carcinogen, suspect human. |
There are many other chlorinated aliphatics that are important solvents, starting with the chlorinated ethane. They can be halogenated with bromine and iodine as well as chlorine. The toxicity seems to come from the metabolism of these compounds,. Often the first step is to remove the halogen, for example
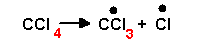 |
That is, it makes two free radicals. |
Then there are the halogenated alkenes. These are metabolized differently than the alkanes. The first step of the alkenes metabolism is to form the epoxide. But first, let's start with a simplest alkene, ethylene.
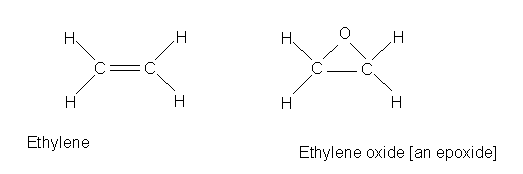
Ethylene is oxidized in industrial processes to ethylene oxide, which is an
epoxide. Ethylene oxide is produced in enormous quantities as a feedstock for
many industrial processes. It is a human carcinogen. The conversion from ethylene
to ethylene oxide does not happen in the humans, so ethylene is not a carcinogen.
Ethylene oxide itself is used itself as a sterilizing agent and thus there is
human exposure.
Vinyl chloride is just ethylene with a chlorine substituted for a hydrogen.
It is also a very common industrial chemical
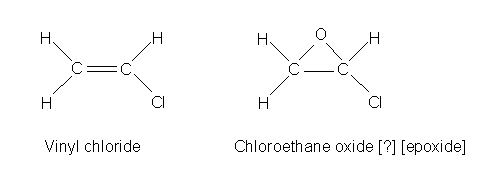
The conversion from vinyl chloride to the epoxide (I'm not sure of the proper
chemical name, extra credit for someone finding that out for us) takes place
in the liver. The epoxide is very reactive. Vinyl chloride is a human carcinogen.
On the other hand trichloroethylene (TCE) is not a carcinogen. One theory is
that the vinyl chloride expoxide can travel further, into the nucleus where
the DNA resides, because it is more stable than the TCE expoxide, which reacts
very quickly with nearby macromolecules. TCE is toxic to the liver and kidneys,
but not a carcinogen. TCE is a very common solvent.
Alcohols
We did ethanol last week. The simplest alcohol is methanol. Methanol is very toxic in humans and primates. It causes blindness and death. Interestingly, it is not very toxic in rodents. This is because alcohols are metabolized differently in the human. Humans change menthanol into formaldehyde which is quickly converted to formic acid, which is the active ingredient in the sting of red ants. This happens on a one to one basis, i.e., one mole of methanol is converted to one mole of formic acid. Humans die of metabolic acidosis. The exact mechanism of the blindness is less certain. There have been incidents of menthanol poisoning where 20 or 30 people die over a weekend. Methanol is not nearly as intoxicating as ethanol, but it smells somewhat the same. Methanol is used as a solvent in some paint thinners. Some paint thinners are drinkable, by the really hard up for a drink of intoxicant. Since the paint or hardware store is selling thinner, they don't post a warning sign when the brand of thinner is changed to one that has methanol. Not everyone dies of course, especially when the word gets out that one of the treatments for methanol poising is administration of ethanol, and the people migrate from under the bridge to the hospital. Some don't make it.
Glycol
Glycols are alcohols, with two alcohol groups (they are diol's).
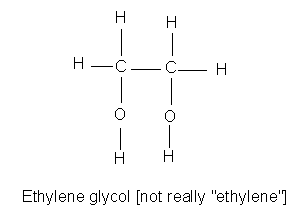 |
The simplest, ethylene glycol (1,2-ethanediol) is quite toxic. It is also quite common, since it is used as an antifreeze. Its metabolism is similar to methanol's and leads to an acid, oxalic acid, which is the toxic ingredient in rhubarb leaves and such. |
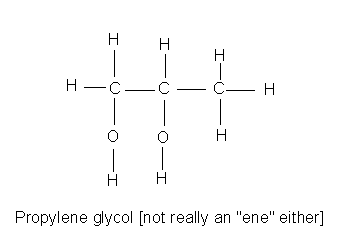 |
Propylene glycol (1,2-propanediol) is quite nontoxic and is used as a food additive. Again, the difference is metabolism, propylene glycol is metabolized to lactic acid which enters the nutrition cycle. |
Glycol ethers
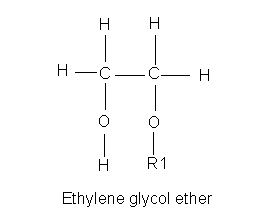 |
Ethylene glycol ether, looks like ethylene glycol, but one of the alcohol groups is an ether, here designated R1. The glycol ethers are common in industrial solvents, lacquers, resins, inks, dyes, and as an antiicing additive to brake fluid and such. They are also toxic to the reproductive system, causing testicular atrophy and infertility in lab mammals. Dioxane (not to be confused with dioxin)is a cyclic diether. It is a widely used solvent and commodity chemical (used to make other chemicals.) It has been responsible for human fatalities, it is a carcinogen, but not a mutagen. It is probably a mitogen. |
Normal Hexane
|
Normal hexane is a simple aliphatic. Normal hexane is just: It is quite neurotoxic. It causes a dying back neuropathy of the axons. |
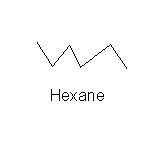 |
Again, it is the metabolic product that is toxic. Normal hexane is transformed into 2,5 hexanedione.
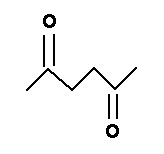 |
The di-ketones, when they are separated by two methyl groups, are very neurotoxic. The other 6 carbon isomers of normal hexane are not neurotoxic. Nor are most aliphatic hydrocarbons. |
So the take-home message is that small differences in the chemical structure may make an enormous difference in toxicity. Some metabolic products are much less toxic, and some are much more toxic, than the parent.
Oh. And why not induce vomiting? Many volatile solvents are toxic to the lungs. If quantities are sucked into the lungs during vomiting, chemical pneumonia follows, i.e., the lungs fill with fluids. This can be fatal. On the other hand, if the solvents are allowed to stay in the GI tract, diarrhea often follows and the solvents are excreted. Even if not excreted that way and some is absorbed, the systemic toxicity is presumed less dangerous.