Covalent bonds and the stick rule
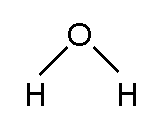
The website you linked to used computer generated "space filling" models to show how atoms are connected. It's easier to show the atoms in a molecule connected by lines or "sticks." Each stick represents a pair of shared electrons. So water is

The "stick rule" just states how many many sticks each element must have.
|
Element
|
Sticks
|
|
H
|
1
|
|
Cl
|
1
|
|
O
|
2
|
|
N
|
3
|
|
C
|
4
|
( Phosphorus and Sulfur are variable, but Phosphorus often has 5 sticks, and sulfur two, in biological compounds.)
So
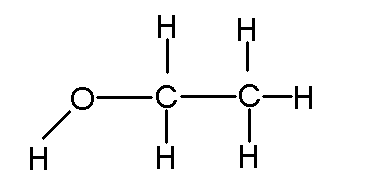
a common recreational chemical, you see each atom has the right amount of sticks. Note that you could have describe the compound as C2H6O. But if you did that, you could have arranged the atoms as such:
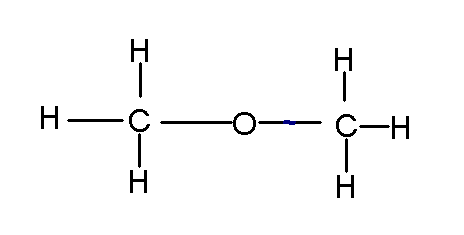
which is dimethylether, a very different compound.
An atom may share more than one pair of electrons with another atom. Here's formic acid, the active ingredient in the sting of red ants
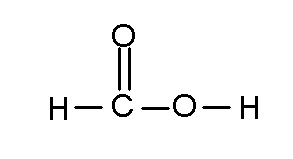
There is a double bond between the topmost oxygen and the carbon. The stick rule still applies. Atoms can bond with other atoms of the same element, and carbon is notorious for this.
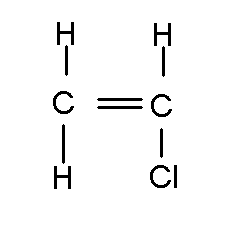
The above chemical is known as vinyl chloride, an vital industrial chemical that is also causes liver cancer in humans. Note how the stick rule applies to all these compounds.