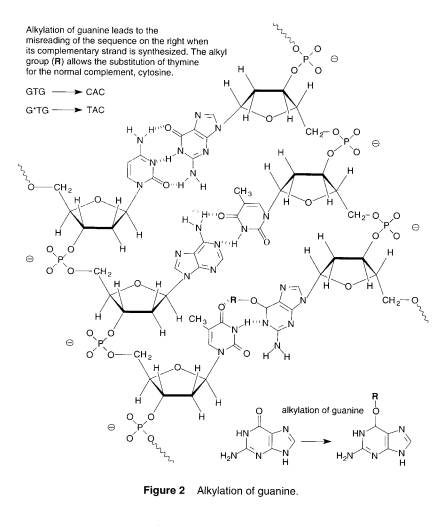
This is from page 110 of your text:

Uggg. Twenty years ago, when my engineering business was prospering in the field of hazardous materials and waste, I decided to try to understand the why of the hazard business. This led me to study industrial hygiene and finally toxicology. But starting out on that quest, I would find items like Figure two above and feel totally incapacitated. Sure I studied chemistry, long, long ago, but then they had only four elements: earth, fire, air, and water. What was "guanine," why would someone want to alkylate it, and what's all the other garbage about it? Anyhow, if you look at Figure 2 above and say, "Of course," you can skip this sub-module and the next ones on chemistry, organic chemistry, biochemistry too. If you said, "Uggg!" the purpose of these modules is to give some familiarity with the key terms and nomenclature such that you are not totally intimidated by things like Figure 2, as well as review some basic chemistry that impacts toxicology.
Basic Chemistry Concepts
Atoms
and Elements. Follow the link to electro-negativity. Note, this should be a review if you've had college chemistry.
Elements and the Periodic Table
Elements are often organized into the periodic
table, which you remember was hanging on the wall of your high school chemistry
lab. Glance at this Periodic
Table and read the Overview. If you want to memorize all the elements, here is a Song that will help you. However for biology, only a few of the elements are "important," meaning you should know them. Here they are biologists periodic table. Thinking back to the electro negative discussion above (you didn't forget already, did you?) Two of the biologically important
are electronegative, oxygen and nitrogen, and these make covalent bonds. Two are very electronegative, chlorine and iodine, usual make ionic bonds..
The periodic tables shown have the atomic number of each element. If you look at the periodic table inside the front cover of your old chemistry textbook you will see that besides the atomic number, it also has the atomic weight. Often we need to know the molecular weight of a compound. This is just the sum of the atomic weights of the atoms in the molecule. Sometimes this sum is given in the units "Dalton." Water has a molecular weight of 18 Dalton. (Back when I went to school, Professor Dalton was still alive, so they called the unit the "atomic mass unit.")
Let's review acids and bases: pH;
Above we discussed how elements are electronegative or the opposite electro-positive - referring to their affinity for electrons. The same principle applies to molecules which are made of elements bonded together. A molecule that has a formal charge is an "ion." That is Na+ is a sodium ion. Someone has borrowed or stolen one of its electrons. (See a little about radicals in a few pages.) However a molecule as a whole can be electrically neutral - have the "right" number of electrons, yet have one part of the molecule that has an excess of charge and another part that has a shortage of charge. We show these charges with a small delta. Molecules that have these charges are known as "polar molecules."
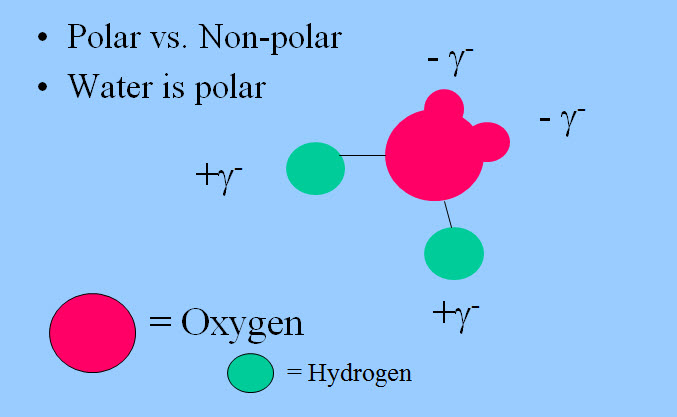
Oxygen, being much more electronegative than hydrogen, pulls the electrons from the hydrogens, or at least the electrons spend more time by the oxygen, and give the molecule a polar nature. Carbon and hydrogen are of about equally electronegative, so the electrons are shared evenly and molecules of only carbon and hydrogen are non-polar.
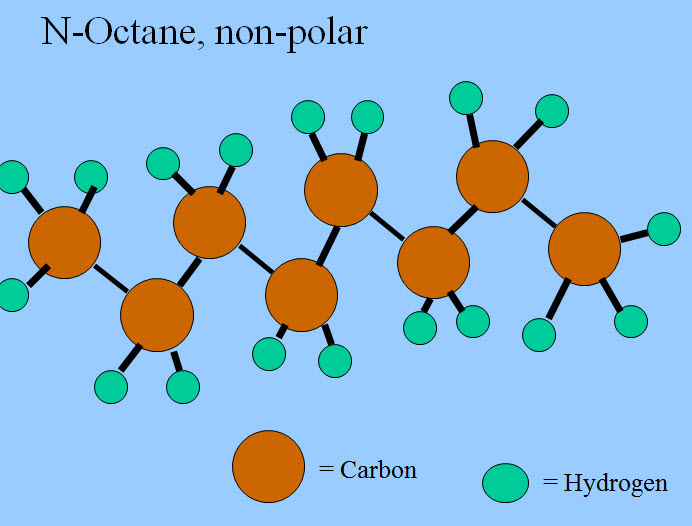
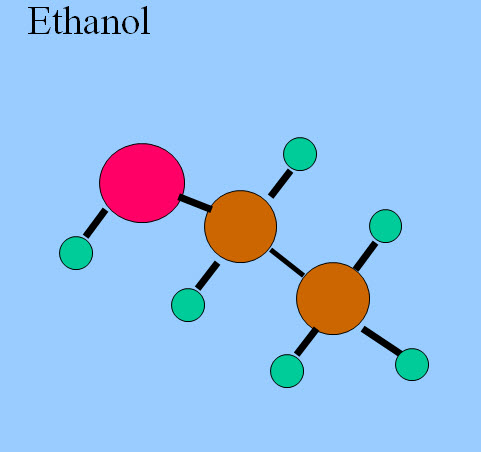
Now "like dissolves like" and polar substances are soluble in water. These polar substances are called "hydrophilic." Non-polar substances are not soluble in water and are known as "hydrophobic." Some substances are both, that is, they have a hydrophilic region and a hydrophobic region. Ethanol is an example.
 |
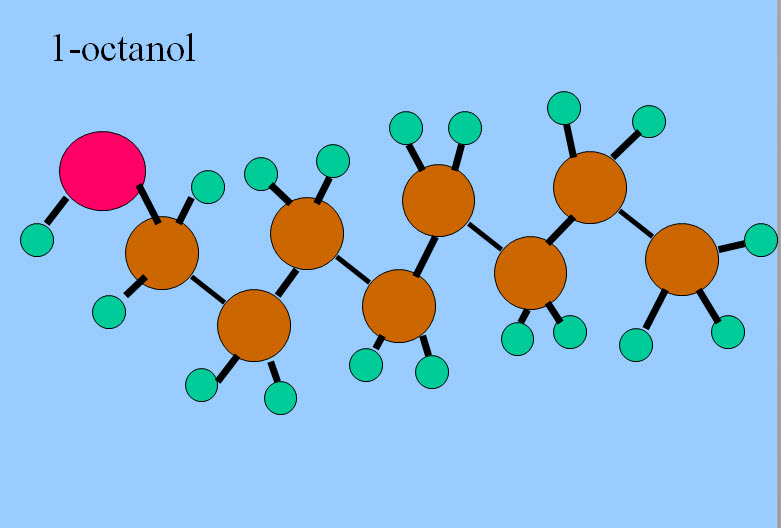 |
Octanol is a very interesting molecule. It has a long, very non-polar hydrophobic hydrocarbon "tail" and a polar hydrophilic alcohol "head." Some substances are "designed" to be both, and we sometimes call these "amphipathic." Soaps and detergents are such, they have a non-polar tail that is absorbed by oily dirt, and a polar head that sticks out into the water. With sufficient agitation, the oily dirt is pulled from the fabric into the water.
If the size of the molecule and their properties are just right, the oil will form a micelles - a micro-droplet of oily stuff surrounded by a layer of amphipathic molecules with their polar heads out towards the water. When I eat a McGreaseburger, the fat is transported in my blood in a kind of micelle called a chylomicron (don't read the whole article) where the oil is the greaseburger and the amphipathic molecules are a collection of special proteins.
With that introduction, we'll start talking a little about cells. Amphipathic molecules of the right characteristics will from a bi-layer. This site has a very good explanation - read down to "Figure 10-7." Be sure to click on the figures.