
Hydrocarbons
The basic hydrocarbon compounds of biological interest consist of carbon atoms linked together, carbon to carbon, with single or double bonds, with the "right" number of hydrogens attached. Now drawing hydrocarbons with sticks and all the "C's" and "H's" get very tedious, even if broken up by an "O" or a "Cl" once in a while. So chemists often use a shorthand of line segments. The chemist mentally places a carbon at each change of direction of the lines (apex) and the ends. Then adds the number of hydrogens that conforms to the stick rule. If there are other elements, they are shown explicitly. So butane, ethanol, and vinyl chloride are shown thus:

So how many carbons are in this compound
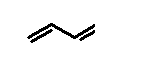 And how many hydrogens
And how many hydrogens
That compound is butadiene (AKA 1,3-butadiene) is an important industrial intermediary in manufacture of rubber and plastics. It is also an irritant and carcinogen.