
Before I send you on to ToxTudor II, which has an excellent presentation on biotransformation and excretion, let me give you an overview. First, let's take a minute to think about some history.

Everyone is fascinated by dinosaurs, but let's consider this picture from the plant's prospective. If the dinosaur is harassed by another dinosaur or a fuzzy little mammal, he can stomp them, or move away. What defense does the tree have? It really only has two defenses - taste repulsive, so animals don't eat it, or poison the animal, so it gets sick and stops eating and perhaps next time the animal remembers that species of tree should not be eaten. OK, so the trees quickly (10 or 20 million years or so) evolve bad tasting buds. Do the herbivorous animals die out? Nope, they evolve some method of digesting the bad buds, and move on. So it has been, actually since long before the dinosaurs, plants evolve toxins and animals evolve defenses against the toxins. Some of the enzymes prominent in biotransformation of xenobiotics are common to vertebrates and insects, groups who parted philogenetic company long before dinosaurs.
Despite the defenses, we can still be harmed by xenobiotics. There are many reasons for this: we don't have the right enzymes for the chemical; we have the enzymes, but not enough of them; the chemical itself destroys the enzymes; or other circumstances, endogenous (age or other disease) or exogenous (concurrent administration of another chemical). Worst of all, biotransformation sometimes makes a more toxic chemical ("product") than the initial chemical ("parent") to which we were exposed. So how does the body eliminate chemicals? A few might be utilized in some beneficial way, perhaps an organic chemical might be burned for energy. But generally xenobiotic chemicals must be excreted, and usually some chemical changes ("biotransformation") are required.
There are many ways a xenobiotic can be excreted, including crying and growing hair, but only a few are important. First the chemicals might be exhaled. This is the common way inert gases, such as anesthetics, are excreted. Small amounts of volatile chemicals, such as ethanol, are exhaled, but only a small amount.
The other two excretion routes are the urine or the feces. Here is a typical sequence: 1.) An organic, lipophilic chemical is inhaled or ingested. 2.) If it is ingested, it is absorbed from the GI tract. 3) Often bound with other lipophilic centers in the blood, the chemical quickly arrives at the liver. 4) It diffuses from the carrier in the blood into cell membrane of the liver cells ("hepatocytes"). 5) The chemical diffuses into the cell via the ER (endoplasmic reticulum - the network of fine tubules within cells). 6) An enzyme in the ER adds an oxygen to the compound ("oxidizes"). The oxygen quickly acquires a hydrogen, so effectively a hydroxyl (OH) group has been added. 7) The process is repeated until the oxidized xenobiotic is more hydrophilic. Steps 6 and 7 are called Phase I reactions. 8a.) If the compound is light, less than 300 daltons, enzymes in the cytosol add some very water soluble compounds, sulfates or a sugar (called glucuronic acid), and the now hydrophilic compound enters the blood to be excreted by the kidneys. Or 8b) If the compound is heavier than 300 daltons, it will will be conjugated with a bile salt and be excreted via the bile into the GI tract and excreted in the feces. The processes of conjugation in Step 8 are called are called Phase II reactions.
There is a different route for compounds such as alcohols that are already somewhat hydrophilic. (We'll get to those in a later module.)
With that, I'll turn you over to the excellent Tox Tudor modules on, Biotransformation and Excretion. (You can skip details in the part about the kidneys in Excretion, we'll do that later.)
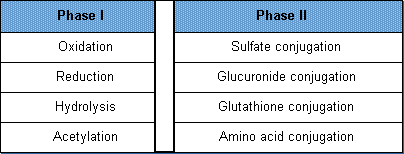
Note a table from Tox Tudor should be two tables, as shown below. The original table implies the Phase I on the left are linked to the Phase II on the right.
The best known and most important Phase I reactions are the oxidation of lipophilic xenobiotics by the "P450" family of enzymes. Like almost everything else we teach, there are other names for the P450's. "Mixed function oxidases" (MFOs) or "CYP" for Cytochrome P450 are some common synonyms. The P450 enzymes are a family with 30 or so subtypes, that all do more or less the same thing. But each subtype has a greater specificity for certain substrates. Cockroaches have P450s not too much different from ours, so the general plan of xenobiotic metabolism has been around for at least half a billion years.
P450s reside in the ER, along with other enzymes and cofactors they need to do their work, which is hanging an oxygen atom onto a lipophilic xenobiotic and making a more hydrophilic chemical. A common lab technique for studies of the P450 and xenobiotics is to crush and centrifuge cells and isolate little globules of ER with the associated P450s and other enzymes and cofactors. These are called microsomes. They are needed in research, because many chemicals are not "detoxified" at all by this process, but rather "activated" or "bioactivated" to more toxic compounds. So the base chemical may be tested, but the result after mixture and processing by the microsomes will also be tested.
Once chemicals are water soluble enough to enter the blood, they may be filtered into the urine or excreted into the urine, both processes we'll talk about in kidney toxicity. If the chemical is heavier than about 300 daltons, it is likely to be conjugated with a bile salt. Bile is essentially a detergent that is excreted into the intestine, just past the stomach, that keeps fat from separating out of the intestine contents. Hanging a toxicant on the salt doesn't affect things much, and the the toxicant-bile salt heads for daylight. Towards the end of the ride, in the colon, there are often bacteria which are capable of hydrolyzing the glucuronide or sulfate to bile salt bond which leave the toxicant free again. If the toxicant is water soluble enough, it continues its ride to the wastewater treatment plant. If it is still lipophilic, it can be reabsorbed in the colon and recycle back through the blood to the liver, to start the process again. Enterohepatic recirculation results in blips on the graph of blood concentration versus time graph, typically 4, 8, 12 hours after administration. The blips become smaller over time.
Many substances are metabolized via different enzymes and pathways. One chemical might have several different "metabolic products," typically most will be benign. But one pathway might lead to activation to a more toxic product. We'll see an example later.
Because of the importance of biotransformation for understanding virtually all drug and toxicant processes, much research has been done and many different processes have been described. You should know about P450s for the Phase I and for Phase II about glucuronidation and glutathione which is described on pages 42 through 46 of your book.