From the Tox Tudor site, look at the two compartment model. Compartment 1 might
be
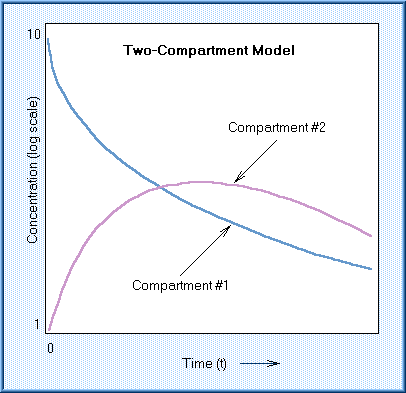
the GI tract, and Compartment #2 the blood. The caffeine concentration decreases
in the GI tract while it increases in the blood. A more common example, compartment
#1 is the blood, say we give a chemical by intravenous injection, and compartment
#2 is the rest of the body. In this picture, and it is commonly true, the compartments
equilibrate, then the concentration decreases in both compartments, often at
the same rate. The reason for this is the chemical is being eliminated from
the body. The slope of those lines can be calculated, and from that slope, k,
the elimination rate constant, can be calculated, or the half life can be measured
and the k calculated from that.
Also, the area under the concentration-time line (curve) can be calculated:
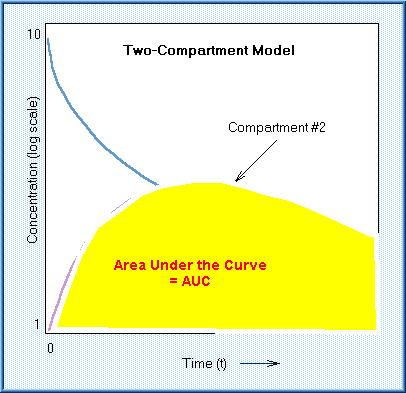
The "AUC" provides a measure of the total availability of the chemical
to the body. Usually, the effect of the chemical is assumed proportional to
the AUC. Chemicals that are eliminated quickly have a short half-life and a
small AUC, for the same dose, than a chemical that is eliminated slowly.
You might have realized that since elimination is driven by enzymes and many
enzymes follows saturation kinetics (aka Michaelis-Menten kinetics) elimination
rate, k, should vary with the concentration. As in this, with a Vmax of 500
and a Km of 100.

Often it does. But in some cases, the substrate concentration is high, compared
to Km; in other cases is is low, compared to Km.
 |
Here we see that once the substrate concentration gets over
about 700, the elimination is approximately constant, regardless of the
substrate. This is called "zero order" elimination. Ethanol at
socially relevant (and dangerous) doses follows zero order kinetics. At
the low end, we see that the line is approximately straight and the velocity
is directly proportional to the concentration This is exactly first order
kinetics. So if the substrate concentrations are low, relative to Km, first
order elimination is a good approximation, even if the the elimination follows
saturation kinetics. |
Clearance and Volume of distribution are important but confusing concepts.
Volume of Distribution is properly termed "Apparent Volume of Distribution"
and has no relation to an actual volume, Vd can be greater than the volume of
the body. It is a method of relating the dose to the concentration measured
in the plasma. Clearance is the volume of blood cleared of the xenobiotic in
a unit of time, usually rationalized on a per kg of body weight basis. Just
remember xenobiotics with a high clearances are quickly eliminated from the
body. Here is a table of some common drugs and their clearance, volume of distribution
and half life. Total body water in a 70 kg adult is about 55 liters.
|
Drug
|
Clearance
L/hour (70 kg adult)
|
Volume of Distribution
(70 kg adult)
|
Half-life (hr)
|
|
Acetaminophen (Tylenol)
|
21
|
66
|
2
|
|
Caffeine
|
5.8
|
42.7
|
5
|
|
Cocaine
|
134
|
140
|
0.8
|
|
Ethanol
|
We'll discuss later
|
37
|
We'll discuss
|
|
Ibuprofen (Advil, etc.)
|
3
|
10.5
|
2
|
|
Morphine
|
100
|
231
|
1.9
|
|
Nicotine
|
76
|
182
|
2
|
|
Theophylline (from tea)
|
2.7
|
35
|
9
|
| |
|
|
|
End of Submodule
Module 3 Index



