Introduction to Stine and Brown, Chapter 4
Protein machines.
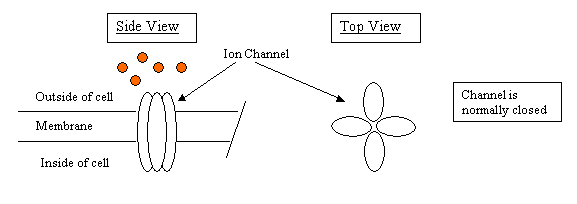
Before you get into Chapter 4, we need to review enzymes, little machines made out of protein that catalyze a biological reaction. But let's start with a protein device even simpler, an ion channel. Here is a channel on the surface of a cell, perhaps a particular type of neuron (nerve cell) in the brain. Its job is to keep chloride ions, the orange dots in the sketch, out of the cell. I've drawn this to show four very similar subunits comprising the channel, many enzymes and ion channels are composed of identical subunits.

Now the body wants to alter the function of that nerve cell, so it secretes a hormone or other messenger into the blood. The messenger finds a special regulatory site on the channel, and binds to that site, perhaps linking to a similar site on the adjacent sub-unit. Here the purple dot is the messenger.
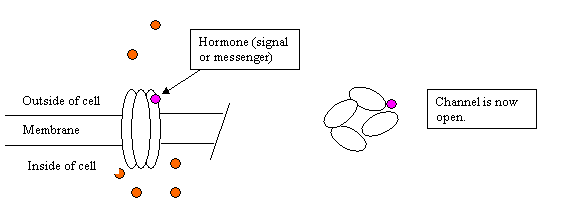
The channel is now open. The site where the messenger binds is called a regulatory site, or an "allosteric site." This channel may have several regulatory sites, perhaps for several different messengers. In other words, different messengers may crank open the channel, with the same or different efficiency. Indeed modulation of the chloride ion channel in parts of the brain is responsible for the effects of many drugs including morphine and ethanol, but the particular regulatory site where those chemicals bind is different.
Now each regulatory site was "designed" for a particular chemical messenger and that messenger fits that site as "a key fits a lock." But there may be other chemicals that also fit the regulatory site. Indeed that is how many pharmaceuticals are developed. They fit a site instead of the usual messenger. The xenobiotic chemical may bind to the receptors with greater or lesser affinity than the biological messenger.
Definition: A receptor is a macromolecule component of tissue with which a drug or chemical ("ligand") interacts to produce its characteristic biologic effects.
Now let's look at enzymes:
Read History for fun and then down to Coenzymes; you might read the rest, but don't need it now. https://en.wikipedia.org/wiki/Enzyme
and the competitive and noncompetitive inhibition from this page Enzyme Inhibition (scroll down).
The names of enzymes usually end in "-ase." They have traditional names, often based on the substrate upon which they work, but sometimes by their product. The traditional name was simple, but not always descriptive, so today enzymes are also known by complex names that more accurately describe their function. Let's not go any further than to recognize that "-ase" means enzyme. This may be a good place to mention that proteins are assembled from amino acids, which are the chief building material of enzymes, but other materials may be involved, such as metal ions, or various organic cofactors. "Lipoproteins" have attachments of lipid and "glycoproteins" have sugar attachments. Ribosomes are a very large conglomerate of protein units and RNA molecular units.
There are two general types of sites on an enzyme that affect its production. The "active site" is the business end of the enzyme where the chemical reaction that is being catalyzed takes place. Then there may be several regulatory or allosteric sites that affect the rate of catalysis.
The authors' goal in Chapter 4 is to acquaint you with several main categories by which xenobiotics affect cells. I'll give you an introduction. But first, let's drop back and look at the big picture for a minute. Very powerful chemicals, such as strong acids, burn flesh. Such gross effects are indeed a "chemical etiology," but their mechanism of action is obvious, and safety rules and common sense protect us. We don't spend a lot of time studying these. But the action of toxic chemicals is often subtle and the mechanisms are not obvious. Very small quantities of chemicals may cause great harm; often the action of these chemicals is the corruption or usurpation of a "natural" process within the body, such a binding to a receptor. Another good reason to learn about the mechanism of action is because toxicology testing is very limited. If we understand the mechanism of action, we are in a better position to predict the effects of the chemicals on different species (we are the species of interest, but almost all the test data is based on lab animals), or effects due to different but related chemicals, or effects at doses much lower than we are able to practically test.
Effects of Toxicants on Enzymes
Figure 4.1 of Chapter 4 illustrates saturation kinetics, a common mathematical model in biology. The [S] on the X axis is the concentration of substrate. Suppose the substrate were glucose and the enzyme's job was to add a phosphate molecule on the glucose, here we further assume there is lots of phosphate and that doesn't affect rate. We measure the rate at which glucose is phosphorylated. The Y axis is the rate ("velocity") of the reaction, in mg/minute. That is, mg of glucose converted to glucose phosphate each minute. You see that the velocity increases until it reaches some limiting value, VMAX. KM is the substrate concentration at which the velocity is half of VMAX. In biochemical experiments of enzyme kinetics it is sometimes possible to determine if a chemical is a competitive inhibitor of the enzyme (that binds to the active site), or a noncompetitive inhibitor (that binds to a regulatory site).
Let's jog to Neurotransmitters and Nerve Gas then come back.
(Continuing to comment on your textbook:) Interaction with (Cell surface) receptors and ion channels.
Transport proteins
Figures 4.5 and 4.6 in Chapter 4 of your book illustrate an important point, using
some enzymes in the mitochondria. Virtually all our energy (and that of all
aerobic organisms) is provided by the breakdown of ATP into ADP. ATP is a high
energy molecule with three phosphate groups. When it gives up one of them, the
resulting energy is used in other chemical reactions to power life processes.
After it gives up one phosphate, it is called ADP, a lower energy compound.
The ADP cycles back to the mitochondria, where energy from the breakdown of
food and oxygen are used to convert it back to ATP. Here is a site that shows
a schematic of the process flow. http://www.brooklyn.cuny.edu/bc/ahp/LAD/C7/C7_atp.html
Your author shows some of the enzymes involved. The process is called the electron
transport chain, and "proton pump." Energy is stored on one side of
a membrane in the mitochondria by a series of enzymes, and in the last step
this energy is transferred to the ATP. Figure 4.6 and the text indicate that four
different toxicants can each block this process, each at a different enzyme.
The effect would be the same, the cell would no longer be able to utilize food
to create energy, but the chemicals and their molecular site of action are different,
although in this case they all bind to a protein. This leads to the next section
of the book
Effects of Toxicant on Receptors and Ion Channels.
Skip page 58 which explains some experimental techniques that might be needed
to sort out the mechanisms, especially details of the binding to the receptor
site.
The end of page 59 through the beginning of page 62 is important, but the author digresses into fine points. A cell, say an epithelial cell in a gland, will have several different kinds of receptors. It will have a receptor for acetylcholine for parasympathetic stimulation (called "cholinergic receptors") and for epinephrine for sympathetic simulation (called "adrenergic receptors," perhaps because adrenaline is a synonym of epinephrine.) But, then the story gets complicated. It turns out there are several types of cholinergic receptors, and several types of adrenergic receptors The different types all respond to the basic stimulation via acetylcholine or epinephrine, but the affinity and cellular responses differ. It was found experimentally that other chemicals bind or affect these receptors, so the subgroups of receptors are known by the experimental chemicals that bind to them, or by numbers. For example the cholinergic receptors have two subgroups, one that responds to the chemical nicotine (a nicotinic cholinergic receptor) and one that responds to the chemical muscarine (a muscarinic cholinergic receptor). But there are subtypes of each of those receptors also.....Let's stop there.
Lipids
Free radicals are capable of destroying intercellular membrane by the process
of lipid peroxidation. We'll go into that in more detail in a later submodule
on free radicals.
Nucleic acids.
We'll spend some more time on the effects of toxicants on nucleic acids next
time when we discuss cancer.
End of Sub-module.