Submodule 8A, page 2
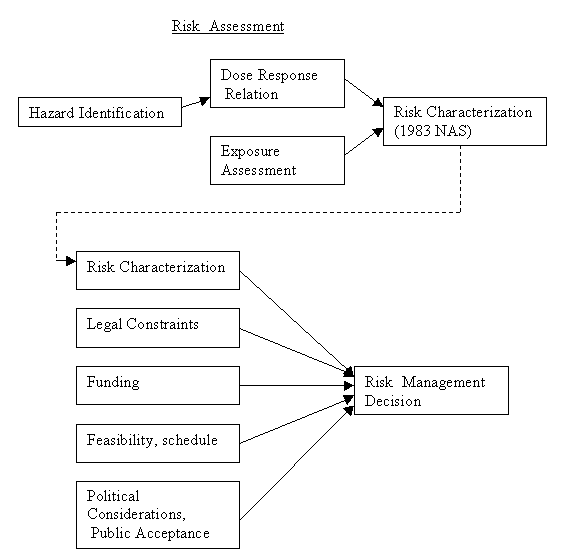
The early 1970's saw an enormous increase in health risk-related laws and regulations. The Occupational Safety and Health Act and the creation of the Environmental Protection Agency were two major laws of that era. By the late 1970's Congress realized that there were many federal agencies evaluating risk, and that they were often using very different standards. Congress commissioned the National Academy of Science (NAS) to study the issue and develop a strategy for evaluating risks. In 1983 the NAS produced Risk Assessment in the Federal Government: Managing the Process. The NAS proposed that the risk process be divided into two phases. The first phase, the Risk Assessment should be separated, completely if possible, from the second phase, Risk Management. The risk assessment would result in a statement of the probability and severity of the harm. Then the risk management phase would decide what, if anything, would be done about the risk. Risk assessment would be a scientific process, unsullied by money and politics. Risk management would start with this scientific input, then consider cost, benefits, politics, and many practical considerations. Here is diagram of the two phases. In technical risk assessment talk, the statement of probability and severity of the harm is called the "risk characterization."

We will talk about some details of risk assessment in 8B, but this submodule is a broad-brush treatment of the kinds of things that should be in each of the phases.
Let's expand each of the four steps in the risk assessment phase (from the 1983 NAS).
Hazard Identification: ...determine whether exposure to an agent can cause an adverse health effect...characterizing the nature and strength of the effect. That is, is the situation worth looking into at all, and if it is, what are we looking for or at?
Dose-Response Assessment:...characterizing the relation between the dose of an agent and the incidence of an adverse health effect.
Exposure Assessment:...estimating the current and future intensity, frequency, and duration of human exposure to an agent.
Risk characterization:...estimating the incidence of a health effect under conditions described in the exposure assessment. (This combines the exposure and dose-response assessment and states uncertainties in the preceding steps.
End of submodule.