Metals
Many metals are toxic. Some metals are essential nutrients. And some metals are both. Besides the fact that the metal atom itself is not biotransformed, the kinetics and biological effects of metals are quite varied. Evaluation and discussion of the toxicity of metals is complicated by several factors. The first complication is that of "species," "oxidation state,"or "valence state," all are synonyms. Most metals of interest are "transition metals" that occupy that middle section of the periodic table. The "stable octet" and the stick rule I gave you earlier work well for the "Main Group" elements of the periodic table.

The transition metals, however, can use electrons in orbitals below the outermost level for bonding, and can therefore share many combinations of electrons.
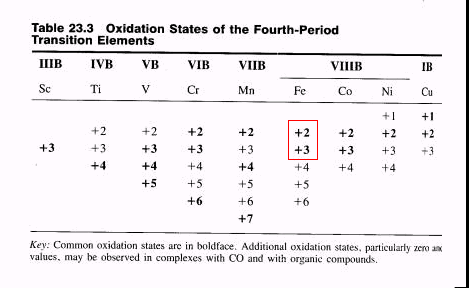
The common oxidation states of iron are ferrous iron, Fe++ or Fe(II), and ferric iron, Fe+++ or Fe(III). The take-home message is that sometimes the toxicity of a metal is dependent on its oxidation state. For example, chrome (III) is common in nature and is an essential nutrient. Chrome (VI) is important industrially and is toxic. Most chrome(III) compounds are soluble, many chrome (VI) compounds are not. Chrome (VI) is a known human carcinogen. Many chrome workers are exposed to both, and many of the environment tests for chrome do not distinguish the oxidation state.
Some metals enter the GI tract as ions in water solutions, others in compounds that are converted to ions in the GI tract (the pH of the stomach is about 2), or are absorbed in the original compound.
Some metals are absorbed from the GI tract and are transported because they mimic a nutrient metal. In that case, toxicity is often seen in the organs that use the nutrient. For example, lead seems to mimic calcium, the CNS uses calcium and lead affects the CNS.
Some metals become lipophilic when they are bound in alkyl compounds, for example methyl mercury or tetraethyl lead.
Here are some effects of lead and tests for lead exposure: http://www.cdc.gov/niosh/docs/98-112/ You should read the first chapter and glance at the rest.
Last topic on metals is chelation. Chelation is the association of a metal ion with a charged or uncharged electron donor known as a ligand. Many ligands form ring structures with the metal, others do not. Many chelation ligands are very nonspecific and will bind many different metals. Several different chelation agents are in use, EDTA (ethylene diamine tetra acetic acid, that it is usually not written with the spaces) is a common treatment for lead poisoning. EDTA binds calcium about as well as lead, so it is given in a calcium salt, where lead will replace calcium and the new molecule will be excreted in urine. EDTA is poorly absorbed from the GI tract, so it must be injected. It is also nephrotoxic, so it is only used for someone who has ill effects from lead.
Here is a table of the "most important" metals, which I would like you to learn about. There is a little in the back of your book about all but chromium. You'll fill out this table for homework.
| Arsenic | Cadmium | Chromium | Lead | Mercury | |
| How are humans exposed? | |||||
| What are oxidation states or common compounds? | |||||
| What are the target organs (or most prevalent problem) | |||||
| Main mechanisms (one or two, if known, mention cell and tissues involved.) | |||||
| What are the kinetics (absorption, distribution, metabolism, and elimination, half life) | |||||
| Is it a carcinogen? Evidence for that? |