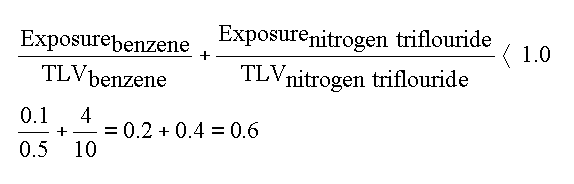
Mixtures
The ACGIH publishes the TLV list. (Review the concept here, if you like, first two paragraphs and the link TLV). The TLV is (to use completely unapproved language) the "safe" limit. So what about mixtures? Here's the basic ACGIH approach:
For example, the TLV for benzene is 0.5 ppm and the TLV for nitrogen trifluoride is 10 ppm. If a worker was exposed to 0.1 ppm benzene and 4 ppm nitrogen triflouride, the industrial hygienist would calculate the fraction of each chemical of its TLV and add these fractions. If the fractions add to less than one, the workplace is within safe limits.
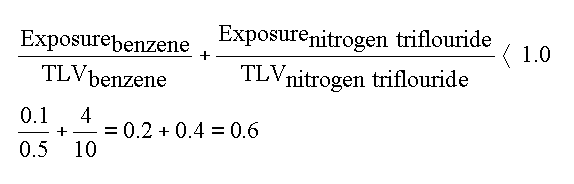
The fractional exposure of each substance is added and if the sum is less than one, the TLV for the mix is not exceeded, and the worker is safe. BUT, the ACGIH goes on to say, "when there is a good reason to believe that the chief effects of the different harmful substances are not in fact additive, but are independent, as when purely local effects on different organs of the of the body are produce by the various components of the mixture." So if the chief effect of benzene were on bone marrow and the chief effect of nitrogen triflouride were not in the bone marrow, the effects could be considered separately (By the way, here's the effects of NF3 from the NIOSH Pocket Guide: "In animals: anoxia, cyanosis; methemoglobinemia; lassitude (weakness, exhaustion), dizziness, headache; liver, kidney injury ." With what you know of benzene, could you consider them separately?
From there, even the ACGIH, which is the practical technical applications of toxicology in the workplace, has to get complex. "Synergistic action or potentiation may occur with some combinations of atmospheric contaminants. Such cases at present must be determined individually. Potentiation or synergistic agents are not necessary harmful by themselves."
Here's some important nomenclature and concepts:
|
Interaction
|
Effects, Symbolically
|
Example
|
| No interaction | A = A and B =B | (Most things.) |
| Additive effects: | A + B = (A + B) | Two organophosphate insecticides, both inhibit acetylcholinesterase. |
| Synergistic effects | A + B = 10*(A+B) | The incidence of lung cancer in asbestos workers is ten times higher in workers that smoked. |
| Potentiation | A (in the presence of nontoxic B) = 10*A | Isopropanol is not hepatotoxic, but when co-administered, it makes carbon tetrachloride much more toxic. |
|
Antagonisms, A (given non toxic B) > A (without B)
|
||
| Functional Antagonism | A raises blood pressure, B decreases blood pressure | Barbiturate overdose leads to sleep, norepinephrine stimulates |
| Chemical Antagonism | A has a chemical reaction with B, might neutralize A | Metal chelation, see next chapter |
| Dispositional Antagonism | B causes faster elimination of A | Increasing or decreasing biotransforming, hydrotherapy to increase methanol elimination |
| Receptor Antagonism (Blockers) | B binds to the receptor where A did its dirty work, but B does not have the effect | Oxygen treatment of Carbon Monoxide poisoning. |
So how do you know, which interaction to expect? The answer is that fully understanding the mechanism of toxicity, including its kinetics, should predict these interactions. But because the mechanisms are not well understood for most toxic substances, the likely effects of interactions are usually not known. Some work is done with some pharmaceuticals and common chemicals.
Potentiation
One mechanism of interaction that is fairly well known relates to the liver
and P450s. "P450s" refers to a superfamily of at least 20 similar
enzymes (called isozymes) that all do more or less the same thing, add an oxygen
to a lipophilic xenobiotic and make it more hydrophilic. Some of the isozymes
seem specific to one or a few chemicals, most isozymes are more general. Most
isozymes are "induceable" by specific chemicals. That is, excesses
of those chemicals somehow interact with the DNA and/or other protein building
machinery and cause more of that isozyme to be manufactured. If increased quantities
of the isozyme are present, Phase I metabolism of chemical will proceed faster.
Depending on the isozyme and chemical, all or only some chemicals will be metabolized
faster. Phenobarbital, a sedative drug, induces most (or perhaps all) isozymes
and is often used to "crank up" the biotransformation rate for experiments.
Now if all the metabolic products of the P450's are less toxic, this induction
is a good thing (pardon the value judgment, it's politically incorrect and scientifically
suspect to use "value laden" terms, like "good" and "bad.")
If some of the metabolic products are bad, that is the parent chemical is "activated,"
then induction will lead to higher levels of the activated product in the tissues,
especially the liver. Alcohols, ethanol and other alcohols, will induce certain
P450's and this may be part of the reason heavy alcohol use is associated with
high rates of many cancers, especially liver cancer.