Alcohol Toxicity
About 80% to 90% of adults consume ethanol. 5% to 10% of adult males and 5% of adult females consume excessive amounts. Ethanol is quite water soluble and is also lipid soluble. It is absorbed unaltered from the stomach and small intestine, and quickly distributed to all tissues. Less than 10% of the total alcohol consumed is excreted in urine, sweat, and breath.
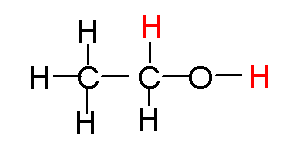
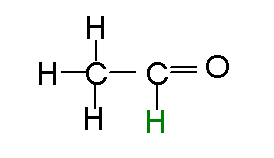
The fist step in alcohol metabolism is the conversion from ethanol to acetaldehyde. In the second step it is converted to acetic acid (acetate).
Ethanol is converted to:

acetaldehyde by three major pathways.
 The predominant pathway
is via a cytosolic enzyme, alcohol dehydrogenase (ADH). The second pathway is
via P450 enzymes, and the third is via a mechanism that uses the enzyme catalase
and hydrogen peroxide. The later is not a predominant pathway in primates, but
is the dominant pathway in rodents. The next step converts the aldehyde to an
acid, acetic acid, which at physiological pH is ionized to acetate:
The predominant pathway
is via a cytosolic enzyme, alcohol dehydrogenase (ADH). The second pathway is
via P450 enzymes, and the third is via a mechanism that uses the enzyme catalase
and hydrogen peroxide. The later is not a predominant pathway in primates, but
is the dominant pathway in rodents. The next step converts the aldehyde to an
acid, acetic acid, which at physiological pH is ionized to acetate:
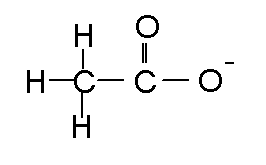
Acetate enters the body's nutrition cycles and is converted to carbon dioxide and water.
People use alcohol because it is a CNS depressant. In non-habitual users, the degree of impairment of the CNS correlates well with blood alcohol level. (Robins). The behavioral effects do not correlate well with habitual drinkers. The literature on the biological and medical effects of alcohol is the largest single literature in medical science (Casarett and Doull's).
Acute effects are mostly CNS related:
|
Blood Alcohol Level (mg/dl)
|
Acute Effect |
|
100
|
"Obvious ataxia" (lack of coordination) (Also the old legal limit for driving, now is 80.) |
|
200
|
Drowsiness |
|
300
|
Stupor |
|
400-500
|
Profound anesthesia. |
Death from CNS depression would be much more frequent except that in steady consumption, sleep usually precedes a lethal dose. In gulping consumption (chugalug) stomach irritation causes vomiting before lethal concentrations are reached. The exact mechanism of the CNS depression is not clear. Of the several mechanisms currently accepted, a general disruption of the cell membranes in the CNS and blocking of certain receptors, are the most widely accepted. My bet is on the disruption of a regulatory (allosteric) site on the chloride ion channel, especially in the dopamineergic and GABA-ergic neurons.
Kinetics
Alcohol is rapidly adsorbed and distributed through the body. Here is a site with information Look at the graph for kinetics and look carefully at the chart. You will note that both axes are linear and the decline of blood alcohol with
time is likewise linear. The ADH enzymes themselves follow Michaelis-Menten
(saturable) kinetics with a Vmax of 124 mg/kg-hr and a Km of 82 mg/l. If you
assume that a liter of blood weighs 1000 grams, you get 0.008%, or one tenth
of the 0.08% which is the legal limit for driving in Alaska. Or look at in another
way, at the legal limit is about ten time higher than the Km. Here is velocity
of elimination at relevant BAC's.
| Blood Alcohol Content , % | V, mg/kg-hr |
| 0.04 | 103 |
| 0.08 | 112 |
| 0.15 | 117 |
| Infinite (Vmax) | 124 |
So at relevant concentrations, rate of metabolism of ethanol is approximately constant. We refer to this at zero order kinetics. This is there reason for the nearly straight line in the graph. If you look again, you will note some of the lines do flatten at very low BAC's which is consistent with M-M elimination.
Breathalyzer.
The concentration of ethanol in the exhaled air is governed by the partitioning
of ethanol between the blood water and the lung air. The number 2100 is used
for this partition, i.e., the concentration in the blood is 2100-fold greater
than the concentration in the air. A lawyer could argue that this number is
not exact and might vary - and it might, slightly, so many states have adopted the 2100 as a legal principle.
The science would be close to that number. Since most of the alcohol remains
in the blood, exhalation is a very slow means of excretion
Chronic Alcoholism and P450 induction
Acetaldehyde is a reactive compound. It is quickly changed into harmless acetate.
The rate of ALDH changing to acetate is faster than the rate ADH changes alcohol
to aldehyde. However in the chronic heavy drinker, the P450 enzymes are induced.
That is, more of the enzymes are available, alcohol is changed to aldehyde at
a faster rate than ALDH can oxidize it. At least that is one theory. There are
many others and the precise mechanics of general damage to the liver, kidneys
and other organs in not known. That contrasts with the effects of that damage,
of which much is known. In addition, poor nutrition, not necessarily associated
with poverty, is responsible for many other effects.
P450 induction.
You recognize that P450's are very beneficial and necessary enzymes. I doubt
we could live a month if somehow all our P450's were shut down. However, a small
percentage of chemicals are metabolized to more toxic products than the parent
compound. We say these are activated. Now the active product is itself
metabolized and excreted, but this takes time and there are only so many enzymes
and processes to do this. The body's repair mechanisms likewise take time and
are saturable. So, if certain P450 isozymes are induced, and a chemical is ingested
that is activated by those isozymes, there will be greater concentrations of
the activated product, than if the isozymes were not induced. Alcohol induces
certain P450 enzymes. Induction of certain P450's is done purposely in many
experiments and many aberrant chemical effects are seen in the induced animals
that are not seen in naive animals. We'll talk a little more about that in our
module on mixtures.
Chronic alcoholism, Alcoholic Liver Disease.
There are three distinctive, albeit overlapping, forms of liver disease. Hepatic
steatosis (fatty liver), alcoholic hepatitis, and cirrhosis. These are collectively
referred to as alcoholic liver disease (ALD)
Within a few days of administration of alcohol to well-nourished, nonalcoholic volunteers, fat appeared within liver cells. The liver transports, processes, manufactures, and stores fat (free fatty acids and glycerides), so there are many proposed mechanism for the fatty liver. Here's a good micrograph of a fatty liver.
Alcoholic Hepatitis
Acute liver necrosis, death of liver cells, causes inflammation of the liver.
Necrotic
cells. Contrast with normal
liver cells. At this magnification, the liver cells are square-ish purple
cells, the nuclei are dark blue or purple. The white lines are the sinusoids.
Also, the presence of mallory
bodies, the dark red bodies in the liver cells that are not present in non-abused
liver.
Cirrhosis Here is a normal liver. What a liver with cirrhosis looks like. Cirrhosis.
Liver enzymes.
One method of detecting liver damage is the presence of certain enzymes in the
blood. Necrotic liver cells lyse (break open) and spill some of these enzymes,
a portion of which are taken up in the blood. Your book mentions several. The
damage might be from any cause, and certain enzymes are more specific to the
liver, some are not specific and might mean damage to another organ. I mention
this here, in the alcohol module, because acute or chronic alcohol overdose
would be far and away the most common cause of these enzymes being elevated.
(Viral hepatitis also produce these enzymes.) However, damage from industrial
chemicals will also cause liver cell necrosis and chemical hepatitis. Often
when workers are exposed to chemicals, especially on some emergency basis, say
a spill and emergency cleanup, and are given a medical exam after the event,
increased levels of these enzymes is taken for proof of excessive exposure to
those chemicals. You can take it from there.