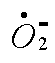
Here is where all the trouble starts:
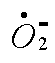
Superoxide anion, aka superoxide, active oxygen, reactive oxygen, etc. We make it in our mitochondria and many intercellular processes including the fabrication of DNA precursors.
An enzyme, superoxide dismutase (SOD), is usually on hand to reduce the superoxide to hydrogen peroxide
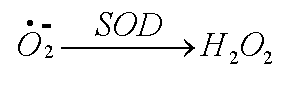
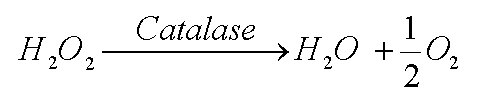
Two good guys are available to get rid of the hydrogen peroxide. The enzyme catalase can convert it to water and oxygen. Or the enzyme glutathione peroxidase can use two glutathione molecules (GSH) to likewise turn hydrogen peroxide into water. (The acronym GSH means the glutathione molecule, but shows the sulfur (S) of that molecule is reduced, that is, has a hydrogen (H) attached (See page 44, 45 of text).
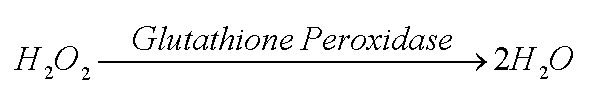 |
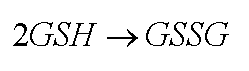 |
Why the hurry to get rid of the hydrogen peroxide? There's a bad guy too.
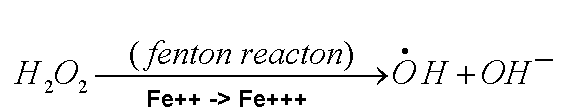
In the presence of ferrous ion, Fe++, hydrogen
peroxide can be converted to hydroxyl free radical.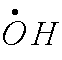 .
Hydroxyl radical is a very bad actor. So superoxide is everywhere, where do
we find ferrous iron? In heme molecules, which are in the red blood cells. So
we would expect to find the protective enzymes, SOD, catalase, and glutathione
peroxidase, in the red blood cells and there are lots of them. But iron and
superoxide are everywhere, so hydroxyl radicals are likewise not uncommon.
.
Hydroxyl radical is a very bad actor. So superoxide is everywhere, where do
we find ferrous iron? In heme molecules, which are in the red blood cells. So
we would expect to find the protective enzymes, SOD, catalase, and glutathione
peroxidase, in the red blood cells and there are lots of them. But iron and
superoxide are everywhere, so hydroxyl radicals are likewise not uncommon.
Here's how hydroxyl free radical makes trouble. Take a lipid molecule in a cell, here I'll draw some of it:
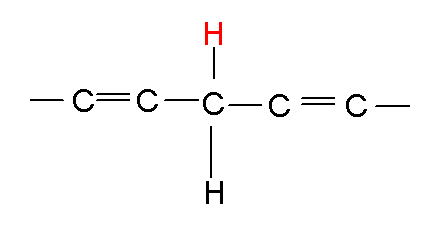 |
Call this LH |
I'll call it L-H, the lipid with the hydrogen its supposed to have, the red one. Now the hydroxyl free radical wants the hydrogen more than the lipid does, so it steals it like so:
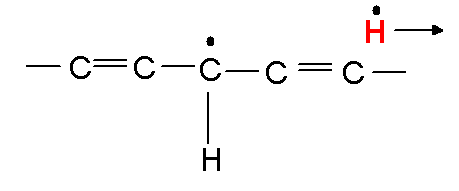 |
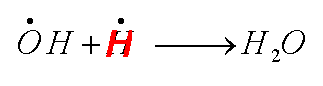 |
| Call the above |
|
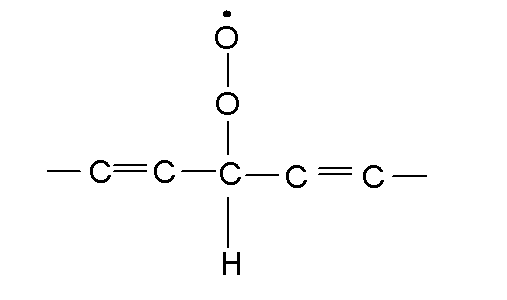 |
Call this 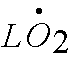 Or
lipid peroxide free radical. Or
lipid peroxide free radical. |
|
The lipid peroxide free radical then steals a hydrogen from a nearby lipid.
|
|
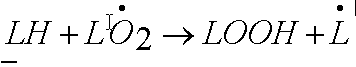 |
The LOOH is now a stable lipid peroxide, but it also made another lipid free radical. |
The lipid peroxide is now a chemically stable molecule, but a different molecule than it was before. It also left a lipid radical, which starts the process again, finds an oxygen, etc. So, one bad radical with the horsepower to start this process can change many lipid molecules into lipid peroxides, thereby changing the nature of lipid wall and barriers within cells, destroying cell membranes.
The process is terminated is when two radicals meet, as in:
|
+
|
=
|
L-L
|
|
|
||
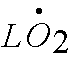 |
+
|
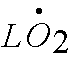 |
=
|
LOOL
|
+
|
O2
|
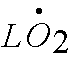 |
+
|
=
|
LOOL
|
|
|
But these are at first very rare so the process of peroxidation can go on and denature many lipid molecules and perhaps destroy a cell.
You may have heard of antioxidants. A common term in the health food industry. Antioxidants, vitamin C, E, and beta Carotene, seem designed by nature to soak up free radicals. They certainly do this in the lab. I don't know of any proof that large quantities of antioxidants taken orally have any effect on antioxidants at the cellular level, or enhance health, for that matter, but supplying antioxidant health foods is a billion dollar industry. Oxidation or free radical damage is associated with the aging process and many of us baby boomers will spend to maintain our youth, or at least keep us entertained while our youth slips away.
Besides detailing another toxic mechanism, you can see how one toxic substance that creates radicals can consume a protective chemical, GSH. The body only has a limited quantity of GSH on hand. It is a time and energy consuming process to add the H back to the GS and make it useful again.