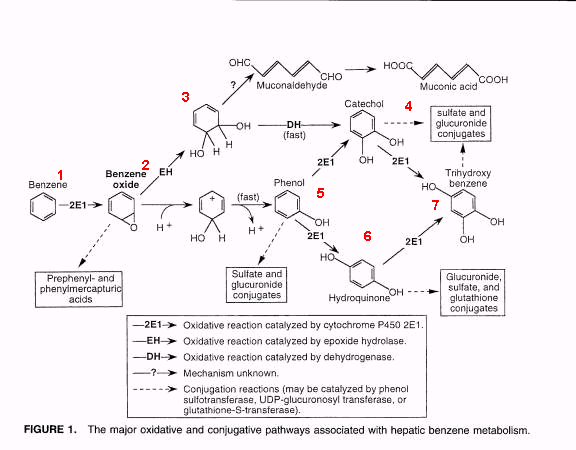
Benzene
Benzene is toxic to bone marrow and causes leukemia. That has been known for many years. It is also a ubiquitous chemical in our industrial society. We are all exposed to it. Let's take a moment to ponder benzene metabolism.
Benzene is absorbed via the skin, inhalation, and orally. It is well absorbed. It is a small lipophilic molecule, but is slightly soluble, more soluble than many common hydrocarbon contaminants.
Here's what happens next:

Copyright CRC, Critical Reviews in Toxicology. 31(3)2001.
1. Starting with benzene,
2. One of the P450 isozymes, 2E1, adds an oxygen. The new compound is benzene oxide, which is an "epoxide;" it is unstable. This seems to be the common primary route of oxidation. There is some evidence that an enzyme can just add an electron to benzene, making a benzene radical. That is not shown.
3. Taking the upper route, an enzyme in the cytosol, epoxide hydrolase, adds a water to the epoxide making a dihydrodiol. By an unknown mechanism, this is converted to muconaldehyde, although the muconaldehyde has never been analyzed in vivo, which is not surprising because the body quickly gets rid of aldehydes. Next the muconaldehyde is converted to muconic acid, probably by an enzyme called aldehyde dehydrogenase. Muconic acid is a common metabolite of benzene, it is a urinary biomarker of benzene exposure.
4. Dropping back to step 3, a cytosolic enzyme, dehydrogenase can convert the dihydrodiol to catechol, a di-alcohol. Catechol may be enter a Phase II reaction and be conjugated with sulfate or glucuronic acid and be excreted in the urine. Alternatively catechol may be further processed by a P450 enzyme into trihydroxybenzene (step 7) and this may enter Phase II
5. Taking the lower route, the benzene oxide may be converted to phenol. The phenol may enter Phase II or it may be converted to either catechol or hydroquinone.
6. The hydroquinone might be converted by a P450 to trihydroxybenzene or it may enter Phase II. There is a glutathion conjugation available to hydroquinone as well as the sulfate and glutathione.
All these metabolic pathways coexist, and result in varied products. The percentage is different in different species. The diagram would show 12 different metabolites, but the possibilities are actually much greater. For many toxic compounds the toxicity is due to a metabolite rather than the parent compound.
It turns out that all of the benzene metabolites of Phase I reactions can be further transformed to benzoquinone
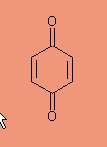 |
Benzoquinone |
and benzoquinone is a direct alkylating agent that forms DNA adducts. It can also cause DNA strand breaks and chromosome damage. Why this damage is so preeminent in the bone marrow is still a mystery. Here is a fairly complete story about the current status of benzene (easy to read article, but it is 6.8 megs to download.). http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1567095/pdf/envhper00391-0033-color.pdf
If you want some details, here is an article that goes into some research issues about benzene, but it's optional.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1469721/pdf/envhper00349-0101.pdf