Particulates
If you 've looked into a shaft of sunlight from the side, you probably noted the many small particles floating in the air. Small particles settle very slowly, and small air currents are sufficient to keep them aloft. Since we breath about 10 cubic meters of air each day, at 1 mg/m^3 of particulates (which is not a terribly dusty environment), we'd absorb 100 g in the first 25 years of life. This much dust would coat the lungs and make breathing more difficult with each year of life. All terrestrial animals have defenses against the dust build up. The nose hairs keep out large particles, like mosquitos. Smaller particles move down the URT and impact the mucus layer, where they stick. The cellular layer beneath the mucus has many ciliated cells that beat and propel the mucus and its dust burden "mouth-ward," where it is swallowed. Our familiarity with mucus (its German word is "Schleim") involves the small amounts that are occasionally expectorated. In fact we swallow about 2 liters of mucus each day. I, unfortunately, got to know lots about mucus when I was developing mass transfer coefficients for some URT research. A lot is known about mucus, because derangement of the mucus is an important aspect in the disease cystic fibrosis. I better stop there, I was about to tell you about harvesting pig mucus, but it's getting late.
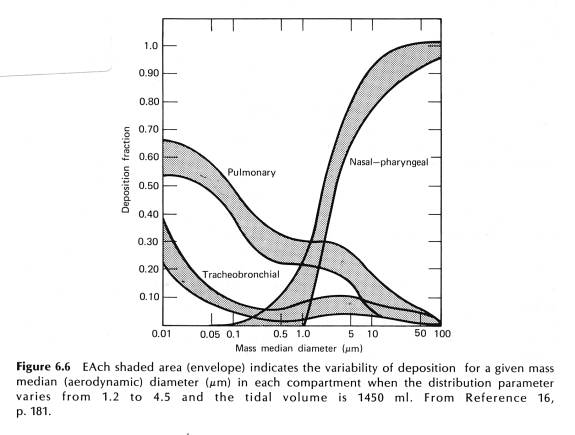
The mechanics of particles interception in the URT is fairly well known. Particles in the airway are entrained in the airstream and some will settle out by gravity. Particles can also impact bifurcations in the airway. The settling of particles is proportional to their diameter. Here is a classic graph of where in the RT particles wind up, versus their diameter.

What this graph says is that about 90% of particles larger than 10 um are deposited in the nose and pharynx; of particles 1 um in diameter, 5% are deposited in the tracheobronchial tree, 5% in the nose, and 30% in the pulmonary regions, that is the alveoli. Importantly, look at particles of size 0.05 um. There you see 60% are deposited in alveoli, where there is no mucus or ciliated cells. So what happens to the small particles? Enter the macrophage:
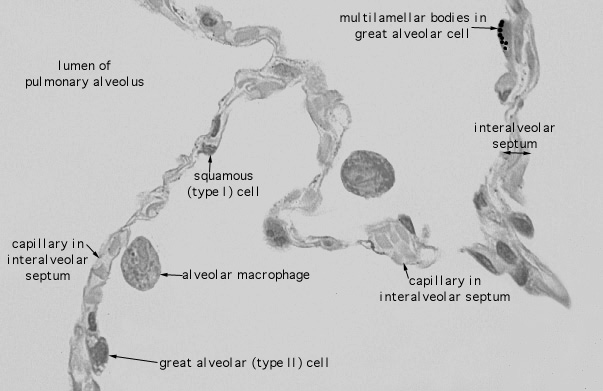
Here's a view of an alveolus, actually several. Note how thin are the walls ("septum") between the alveoli. For scale, the diameter of the macrophage is about 7 um.
Here is a color micrograph. Note the reddish colors are due to the staining technique. The dark color of the macrophage ("M") is due to dust inclusions.
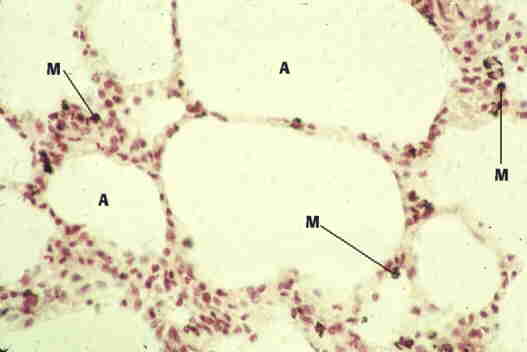
Photo copyright, Elizabeth K. Perryman.
So what happens to the macrophages when they are "full?" They crawl (actually motivate using pseudopodia, kind of like an ameba) and work their way towards the URT. At some point they hop on the mucociliary escalator, and....hope it doesn't spoil your lunch. Some will try a different route and find their way to a lymph duct and exit the lungs that way.
Since a macrophage is about 7 um in diameter, it can "swallow" 1 or 2 um diameter particles, dust or a bacteria, with no problem. So the system works great, larger particles settle out in the URT and smaller particles get swept up by the macrophages. End of story? Nope. There are two more problems I want to tell you about that are very important to toxicology of the RT, and both involve the macrophage.
First problem is that the diameter mentioned above is the aerodynamic diameter. For a long thin particle, called a "fiber," the aerodynamic diameter is approximately the diameter of the particle, not its length. So a long thin fiber will penetrate much deeper in the lungs than a roundish particle of the same weight. From the topmost graph, if a particle were 15 um long and only 0.05 um in diameter, most of the fibers would reach the pulmonary region of the lung. Once there, however, they would be twice as long as the macrophage that is assigned to scarf them. Macrophages are designed to ingest biological particles such as fungal spores and bacteria. Once inside, the macrophages pours deadly agents on them, especially some powerful oxidizing agents . That works great for a particle that is fully "ingested" and placed in an appropriate organelle inside the macrophage. For a long fiber, however, the process gets very sloppy and the oxidizing agents, including hydrogen peroxide, leak into the surrounding lung tissue. This generates free radicals (which we'll get to in a future module) especially if there is iron available, which there is, in many forms of asbestos. This process leads to chronic inflammation of the lungs, asbestosis, and cancer.
Silica is also toxic, but by a different mechanism. Silicone dioxide is quartz, the chief ingredient of most beach sands. Certain crystalline forms of quartz, when ingested by the macrophage, are toxic to them, i.e., it kills the macrophage. This would not be so bad, except the quartz is still there, now with a dead macrophage to boot, so another macrophage must come by to clean the mess up, and the process repeats itself. Eventually the particles gets coated with enough gunk so that it can be disposed. However, besides being tiny shopvacs, macrophages play an important role in the immune system. During the process of battling with the silica particles, in a certain percentage of the exposed humans, an autoimmune reaction is set up. This leads to chronic inflammation and often death of workers highly exposed to silica. Sandblasting was a worst case, the process produced in many fine particles This site has a good overview of occupational lung diseases. The formal name for dusty lungs, or the inflammatory reaction to them, is pneumoconiosis, the pronunciation of which will be on your oral final. (Just kidding, but it's "new moe' coe nee oh sis").
End of submodule