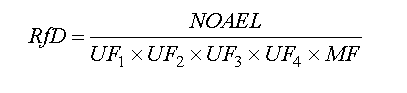
Non-cancer effects
The rationale for evaluating the non-carcinogenic effects is the assumption that they have a threshold. That is, a dose below which there are no adverse health effects. That threshold is referred to as the reference dose (RfD). The RfD is the amount of xenobiotic per kg of body weight per day that is not expected to result in adverse health effects. Here is how the RfD for humans is calculated: Take the NOAEL (or LOAEL) from animal studies and divide by some uncertainty factor. (You remembered that NOEL stood for no observed effects level. NOAEL means about the same thing and stands for no observed adverse health effects. LOAEL is similar and is the lowest dose when harmful effects were noted.) Here is a scientific-looking equation:
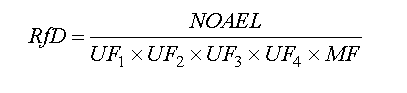
UF1 Uncertainty Factor due to extrapolation from animal data
UF2 Uncertainty Factor due to application to sensitive human populations
UF3 Uncertainty Factor due to use of sub-chronic instead of chronic NOAEL
UF4 Uncertainty Factor due to use of LOAEL instead of NOAEL
MF Modifying Factor allows for professional judgment of additional uncertainties
(The number used for subscripts are not important)
The UF for extrapolation from animal data is usually given a value of 10. The UF for evaluation to sensitive human population is also given a value of 10, for most environmental studies. So the first two uncertainty factors yield a combined uncertainty factor of . That means if the NOAEL in the rat study was 50 mg/kg day, the RfD would be . The next uncertainty factor refers to the length of time the rats were exposed. If the test lasted 24 months (a rat's typical lifetime) the UF would be 1 because the tests were chronic. If the test lasted less than 2 months, the test was non-chronic and the value used here would be 10. The next UF would be 10, if the rat study did not find a NOAEL and had to use the LOAEL. That means that adverse effects were observed in the lowest exposure dose. Lastly the MF or modifying factor (fudge factor) is 1, if the scientists (or agency promulgating the RfD) has high confidence in the data. For example if several different, high quality laboratories developed the same results. Conversely, if the data appeared inconsistent, or there was little data, or the data was otherwise suspect, a MF of 10 might be applied. So the product of the uncertainty and the modifying factors is always at least 100 and may be 100,000. So if chemical Z has a LOAEL of 60 mg/ kg day in the only rat study done, a two week study, and that study had inconsistencies, what would the RfD be. Any of UF's or the MF might be a number between 1 and 10, based on the judgment of the person deriving the RfD.
Depending on your source for the RfD, you may find several for the same chemical. For example the RfDo for oral, sometimes RfC is used for reference concentration for inhalation exposures. The RfD that you use will have to correspond to the risk you are evaluating. We will work more with this via examples from IRIS in a few weeks. (IRIS, Integrated Risk Information System, is an EPA database.) Often there is exposure via several routes, for example in Module 5 the kids had exposure via both the dermal and inhalation route. We will discuss how to combine these next week. NEXT
BACK ENVE 651 Home Module 6 Index