Sub-module 5A, chem lesson, page 3
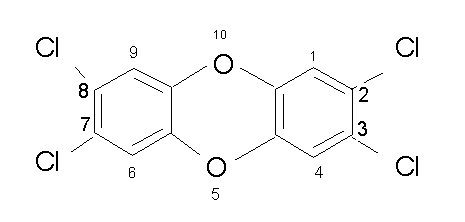
Dioxin is the media name for this chemical.

The locations where the hydrogen atoms go are numbered. If the four chlorines were not there, I'd call this dibenzo[b,e][1,4]dioxin, because that's what the books say to call it. Most folks call it dibenzo[p]dioxin, but I have no clue where the [p] came from. Now if you start replacing the hydrogen atoms with chlorines, you need to describe where the chlorine are. The compound above is 2,3,7,8-tetrachlorodibenzo[p]dioxin. Or TCDD. You don't need to put the numbers before the TCDD if you are talking to a toxicologist. The 2,3,7,8 is assumed and is one of the most toxic compounds ever studied, in the guinea pig. The LD50 is 0.0006 mg/kg. It is also an animal carcinogen and suspected human carcinogen. It also causes birth defects, etc.
Now here is an isomer 2,3,6,7 TCDD. This substance would have the same molecular weight and similar chemical properties, but is not nearly as toxic as TCDD, if it is toxic at all.
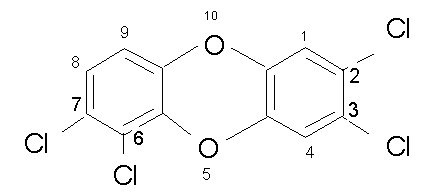
Why? The reason is that TCDD works by fitting into a special chemical receptor in the cell. These receptors require a precise fit. If TCDD fits exactly, the other is not likely to fit. There are other combinations of chlorine that will fit, and these are given a rating relative to TCDD. You can glance through the TEF s about page 13. We will spend a more time with these in our "special chemical" module.